PERVATECH BV, established in 1999 is a leading company in membrane technology. Based on the belief that we have an obligation towards future generations to preserve Earth’s resources, we develop products and services that enable customers to innovate their products and production processes towards lower energy consumption, less waste and higher quality. We produce membranes, membrane modules and systems for pervaporation and vapor permeation applications. In this case study we present energy efficient methods to retrieve acetonitrile from waste streams, please contact us for more detailed information.
Case study
Acetonitrile is a popular solvent in the petroleum industry for the purification of butadiene and as a common mobile phase in liquid chromatography. Acetonitrile is formed as a by-product during the production of acrylonitrile and is therefore directly related to the acrylonitrile production. In 2008 a shortage of acetonitrile occurred due to a production stop for the Olympics in China and a damaged production facility in the US because of hurricane Ike.
In order to make companies less dependent on acetonitrile producing companies and to decrease acetonitrile rich waste streams, Pervatech BV did a study on the dehydration/purification of acetonitrile. One way of recycling is the use of distillation where the azeotrope, xacetonitrile=0.837, is the limiting factor in the dehydration process. The azeotrope can be broken by the use of pressure swing distillation or by extractive distillation. Since these kind of separation processes are quite complex and often an extra separation step is necessary to remove the entrainer liquid, a simpler separation process is designed that is cost and energy efficient.
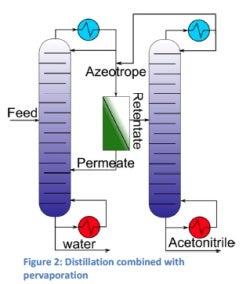
For the dehydration / purification of acetonitrile, different solutions are possible. One of these solutions is to combine distillation with pervaporation in order to break the azeotrope. Another solution is the complete dehydration of acetonitrile by pervaporation; due to the large membrane area required this isn’t an economic solution for large streams. For small streams on the other hand this can be a promising solution.
Additionally, the dehydrated acetonitrile can be purified using membranes to get the required purity for the process where it is being used. The dehydration of acetonitrile by the combined distillation and pervaporation was worked out as a case study for a 8,000kg a day plant. The results obtained show the potential of this environment-friendly dehydration process where the mixture is first distilled to azeotropic composition, then the azeotrope is broken by pervaporation and the retentate is led to another distillation column where the acetonitrile is distilled further (figure 2).
In order to calculate the required membrane area for breaking the azeotrope, dehydration experiments were carried out with different membranes, water mass fraction of 0.2 until 0.001, in a batch wise process. The membrane areas calculated from the experiments are stated in table 1 for both the HybSi® and Silica membrane. The HybSi® membrane shows the smallest required surface area for the separation but also the highest permeation of organics.
Conclusion

The dehydration of acetonitrile for recycling/purification can be optimized by:
- 1) The use of ceramic membranes for breaking the azeotrope
- 2) Full dehydration of acetonitrile from the azeotropic point up to specification: residual water < 0,5 – 0,1%
For 1) The HybSi® membrane is preferred because of its high flux, despite the higher organic content in the permeate, which has to be fed back in the distillation column. For 2) The Silica membrane is the best choice for the complete dehydration of acetonitrile, because of the high selectivity and therefore low permeation of organics.








